Background: Liso-cel, an autologous, CD19-directed, 4-1BB CAR T cell product administered at equal target doses of CD8 + and CD4 + CAR + T cells, has previously demonstrated clinically meaningful responses in patients with R/R FL in the global phase 2 TRANSCEND FL study (NCT04245839). Limited evidence is published on estimates of health care resource utilization (HCRU) and cost of postinfusion care among patients with large B-cell lymphoma (LBCL) treated with liso-cel (Palomba ML, et al. Leuk Lymphoma 2021), and no evidence is currently available for patients with R/R FL. This analysis evaluated postinfusion HCRU and estimated cost of care across a 6-month period among patients treated with liso-cel in the TRANSCEND FL study.
Methods: In this retrospective analysis, patient-level HCRU data from the TRANSCEND FL study database, including hospitalizations and length of hospital stay (LOS) for the entire hospitalization period postinfusion (standard inpatient [IP] and ICU days), diagnostics (eg, laboratory work and imaging), procedures (eg, dialysis and intubation), and medications (eg, oncology supportive care, prophylactics, and other AE management), were analyzed from the day of liso-cel infusion through 6 months of follow-up. Postinfusion costs (not including the cost of liso-cel) were estimated using a microcosting methodology in which HCRU in the 6-month period was identified and unit costs sourced from public databases or literature (adjusted to 2023 US dollars [USD] using the Consumer Price Index) were applied to each HCRU. Facility costs were sourced from the Healthcare Cost and Utilization Project National Inpatient Sample (2020) databases (Dasta JF, et al. Crit Care Med 2005), and the US Centers for Medicare and Medicaid Services (CMS) Hospital Outpatient Prospective Payment System (OPPS; 2023). Diagnostic and procedure costs were obtained from the CMS OPPS (2023), clinical laboratory fee schedule (2023), physician fee schedule (2023), durable medical fee schedule (2023), and Dasta 2005. Medication cost data were obtained from IBM ® Micromedex ® RED BOOK ® (2023) using wholesale acquisition cost and were uniformly applied across sites of care. Average total cost by month after liso-cel infusion was calculated among patients with ongoing status in that month. Patients censored due to data cutoff were excluded.
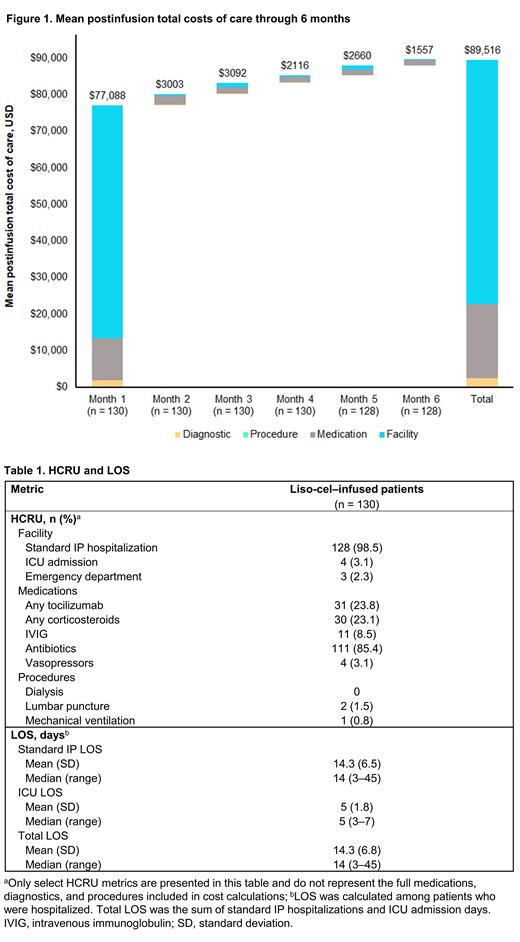
Results: A total of 130 patients were treated with liso-cel in the TRANSCEND FL study, primarily in the fourth-line setting (45.4%). These patients had a median (range) age of 60 (23‒80) years and were predominantly male (83/130 [63.8%]) and White (69/130 [53.1%]); 45 (34.6%) had unknown race. Among patients who received liso-cel infusions, the estimated mean 6-month postinfusion total cost of care was $89,516 (Figure 1). Most 6-month costs ($77,088 [86.1%]) were incurred in Month 1 after infusion and were primarily driven by facility costs ($66,683 [74.5%]), namely standard IP hospitalization and ICU stays. HCRU and LOS (including standard IP hospitalization and ICU days) are shown in Table 1. Almost all patients were hospitalized in the standard IP setting (128/130 [98.5%]). Few patients were admitted to the ICU (4/130 [3.1%]), and 3/130 (2.3%) had an emergency department visit. Median (range) hospitalization time was 14 (3‒45) days for standard IP and 5 (3‒7) days for ICU stays. Some patients received corticosteroids (30/130 [23.1%]) or tocilizumab (31/130 [23.8%]), and few required intravenous immunoglobulin (11/130 [8.5%]), vasopressors (4/130 [3.1%]), lumbar puncture (2/130 [1.5%]), or mechanical ventilation (1/130 [0.8%]). No patients received dialysis.
Conclusions: The majority of costs in the 6-month period after liso-cel infusion among patients with R/R FL in the TRANSCEND FL study were incurred in Month 1 and were facility related (IP and ICU hospitalizations and LOS). These findings, consistent with those previously reported for patients with LBCL treated with liso-cel, support that a single infusion of liso-cel is associated with significantly less HCRU and costs after the initial treatment period in the first month.
Disclosures
Saunders:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Singh:Bristol Myers Squibb: Research Funding. Lee:Bristol Myers Squibb: Research Funding. Farazi:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Fasan:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company; Sanofi Genzyme: Speakers Bureau; Oncopeptides: Other: Advisory Board, Speakers Bureau. Mirza:BluePath Solutions: Current Employment; Bristol Myers Squibb: Consultancy. McGarvey:Bristol Myers Squibb: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal